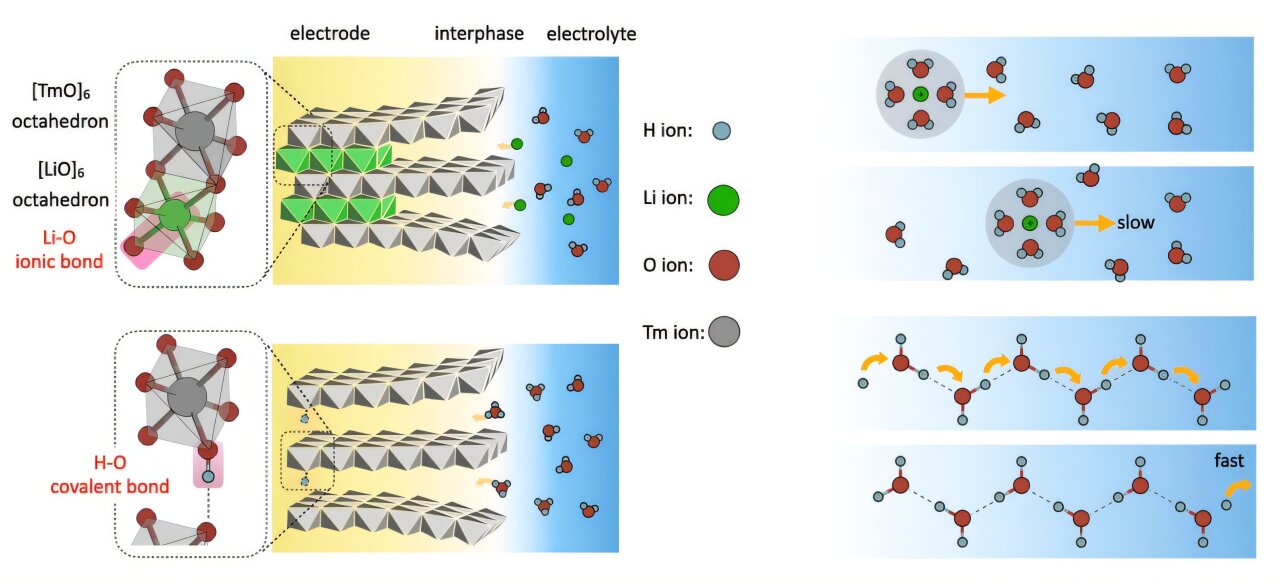
According to foreign media reports, a research team led by Professor Pan Feng from Peking University Shenzhen Graduate School’s School of Advanced Materials has revealed the key mechanisms of proton storage and transfer in aqueous batteries. This research provides significant insights, aiming to develop safer, faster-charging, and higher-capacity alternatives to lithium-ion batteries. The study, titled "Proton Storage and Transfer in Aqueous Batteries," published in the journal Matter, demonstrates how hydrogen bond network engineering can achieve efficient proton storage and transfer. Aqueous batteries using water-based electrolytes are inherently safer than lithium-ion batteries, though they have traditionally had lower energy density. Protons, due to their light mass and high mobility, hold great promise, but their complex chemical properties have limited practical applications. Professor Pan’s team has shown that protons move via a Grotthuss-like mechanism, hopping between hydrogen bonds rather than diffusing like metal ions. This enables ultra-fast, 'non-diffusive' transport, making protons ideal charge carriers for high-performance aqueous batteries.
New Research Unveils Key Mechanisms of Proton Storage and Transfer in Aqueous Batteries
Share this post on: